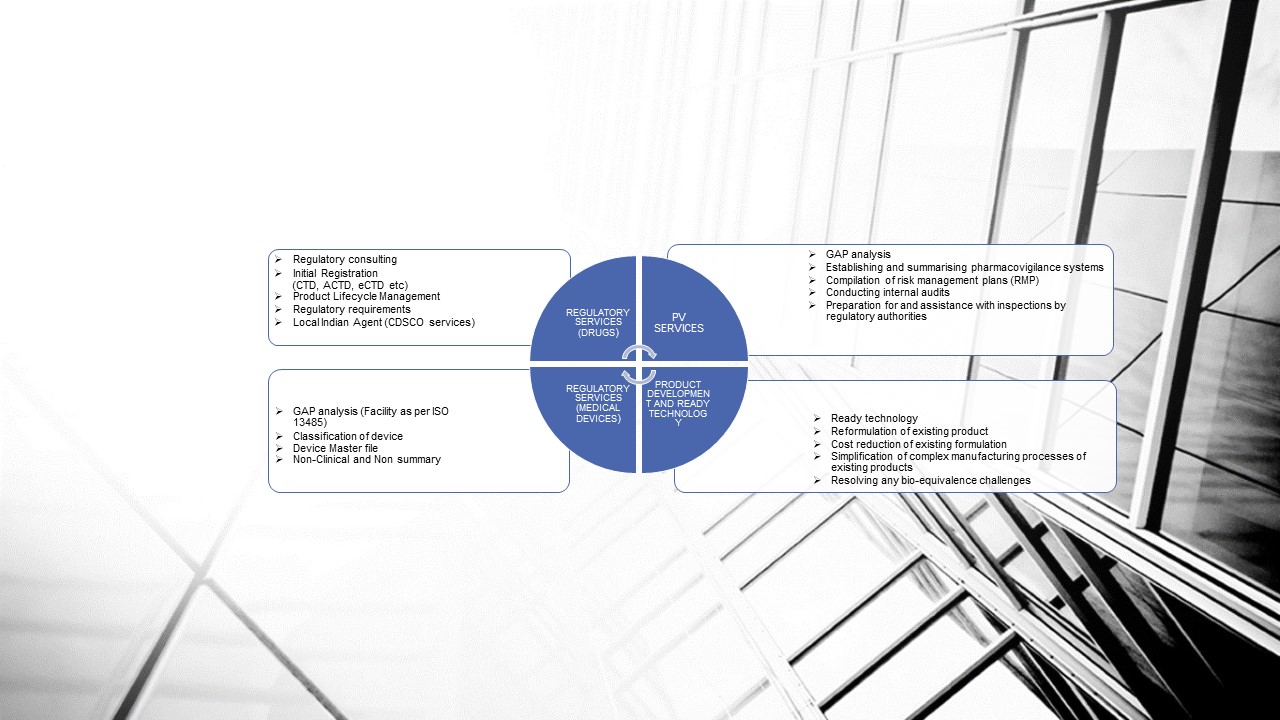
REGULATORY SERVICES FOR INDIAN MARKET (Medical Devices)
In India, at present only notified medical devices are regulated as Drugs under the Drugs and Cosmetics Act 1940 and Rules made thereunder in 1945.
-
Substances used for in vitro diagnosis and surgical dressings, surgical bandages, surgical staples, surgical sutures, ligatures, blood and blood component collection bag with or without anticoagulant covered under sub-clause (i);
-
Substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings), disinfectants and insecticides notified under sub-clause (ii); and
-
Devices notified from time to time under sub-clause (iv), of clause (b) of section 3 of the Drugs and Cosmetics Act, 1940;
INVITRO DIAGNOSTICS:
In India, import, manufacturing, sale and distribution of In Vitro Diagnostic Medical Devices are regulated as Drug under sub-clause (i) of clause (b) of section 3 and sub-clause (iv) of clause (b) of section 3 of Drugs and Cosmetic Act and Rules.
The requirements for grant of licence to Manufacture, Import, Clinical Performance, Sale and Distribution are prescribed in MDR-2017.In vitro diagnostic medical devices shall be classified on the basis of risk parameters as specified in Part II of the First Schedule, as under:
-
Low risk - Class A;
-
Low moderate risk- Class B;
-
Moderate high risk- Class C;
-
High risk- Class D.
Classification list of In Vitro Diagnostic Medical Devices have been published on the website of the Central Drugs Standard Control Organisation approved by CLA.
The Central Licensing Authority shall be the competent authority for enforcement in matters relating to:
-
Import of all Classes of In Vitro Diagnostic Medical Device
-
Manufacture of Class C and Class D In Vitro Diagnostic Medical Devices
-
Clinical Performance evaluation and approval of new in vitro diagnostic medical devices
-
Co-ordination with the State Licensing Authorities
The State Drugs Controller, by whatever name called, shall be the State Licensing Authority and shall be the competent authority for enforcement in matters relating to-
-
Manufacture for sale or distribution of Class A or Class B In Vitro Diagnostic Medical Devices
-
Sale, stock, exhibit or offer for sale or distribution of In Vitro Diagnostic Medical Devices of all classes.