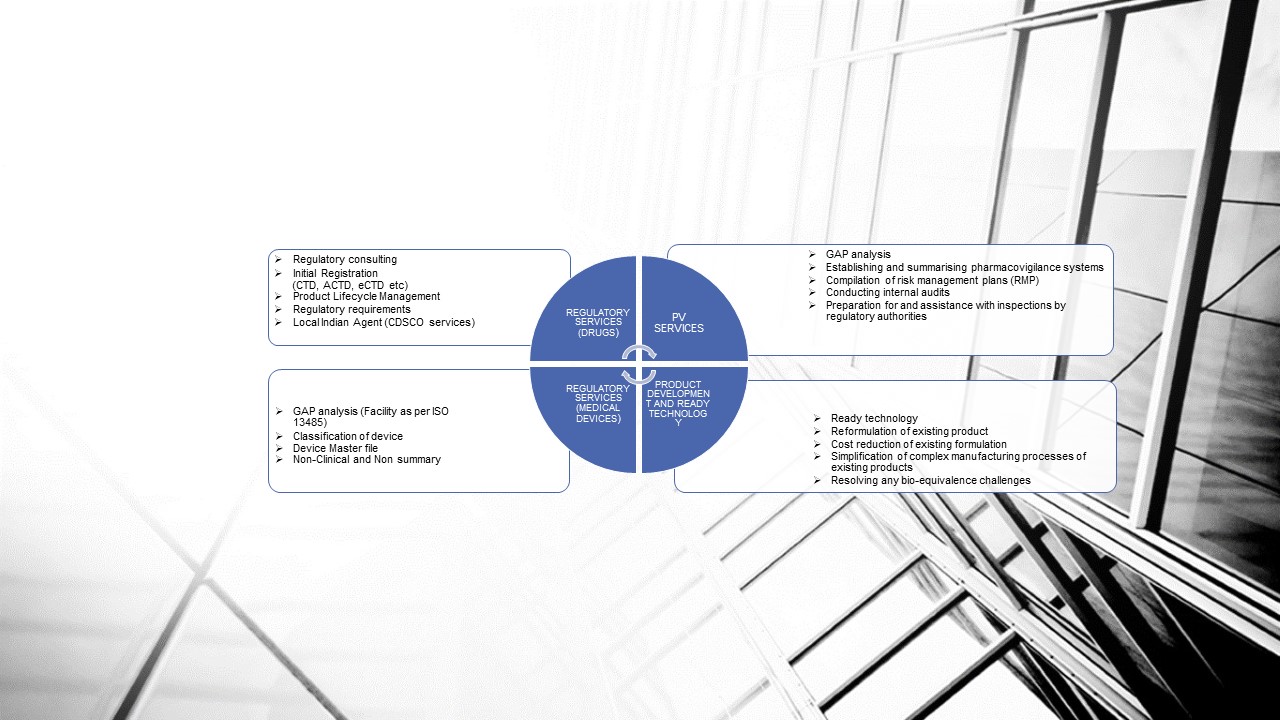
REGULATORY SERVICES FOR INDIAN MARKET (Cosmetics)
As per Section 3(aaa) of Drugs and Cosmetics Act, 1940 “Cosmetic” means “any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applied to, the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance and includes any article intended for use as a component of cosmetic.”
Under the provisions of Drugs and Cosmetics Act, 1940 and Rules made thereunder, the manufacture of cosmetics is regulated under a system of inspection and licensing by the State Licensing Authorities appointed by the respective State Governments while the import of cosmetics is regulated under a system of registration by the Licensing Authority appointed by the Central Government. The Drugs Controller General (India) functions as the Licensing Authority who grants the registration certificate and regulates the import of cosmetics in India vide Gazette notification G.S.R 426(E) under the provisions of Drugs and Cosmetics Act, 1940 and Rules made thereunder.