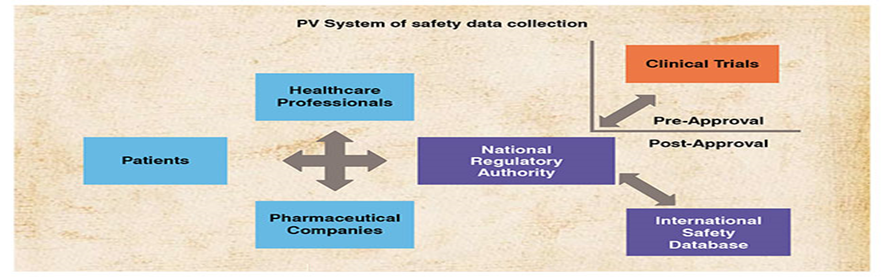
Pharmacovigilance
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problem.
The aims of PV are to enhance patient care and patient safety in relation to the use of medicines; and to support public health programmes by providing reliable, balanced information for the effective assessment of the risk-benefit profile of medicines.
A pharmacovigilance outsourcing company should enable its clients to interact with one service provider for all the Pharmacovigilance (PV) and regulatory requirements, which translates into a cost and time effective strategy.
PV SYSTEM ESTABLISHMENT:
Medwisdom provide the best services to develop the pharmacovigilance system through the following activities:
-
Evaluation of existing pharmacovigilance activities (SOPs, literature research, reporting, PSURs, alarm plans, etc.) and measures to minimise risk
-
Establishing and summarising pharmacovigilance systems (pharmacovigilance system master file, PSMF) for pharmaceutical companies, including annexes
-
Compilation of risk management plans (RMP)
-
Conducting internal audits
-
Preparation for and assistance with inspections by regulatory authorities
SAFETY DATA BASE
We have an association with the safety database provider. We provide safety database at very competitive rate. Which is fully validated E2B (R3) compliant safety database, which enables quality and efficiency in handling safety data. In accordance with Good Clinical Practice (GCP) and Good Pharmacovigilance Practice (GVP), this allows continuous evaluation of the risk-benefit profile of products throughout clinical and post-marketing development.