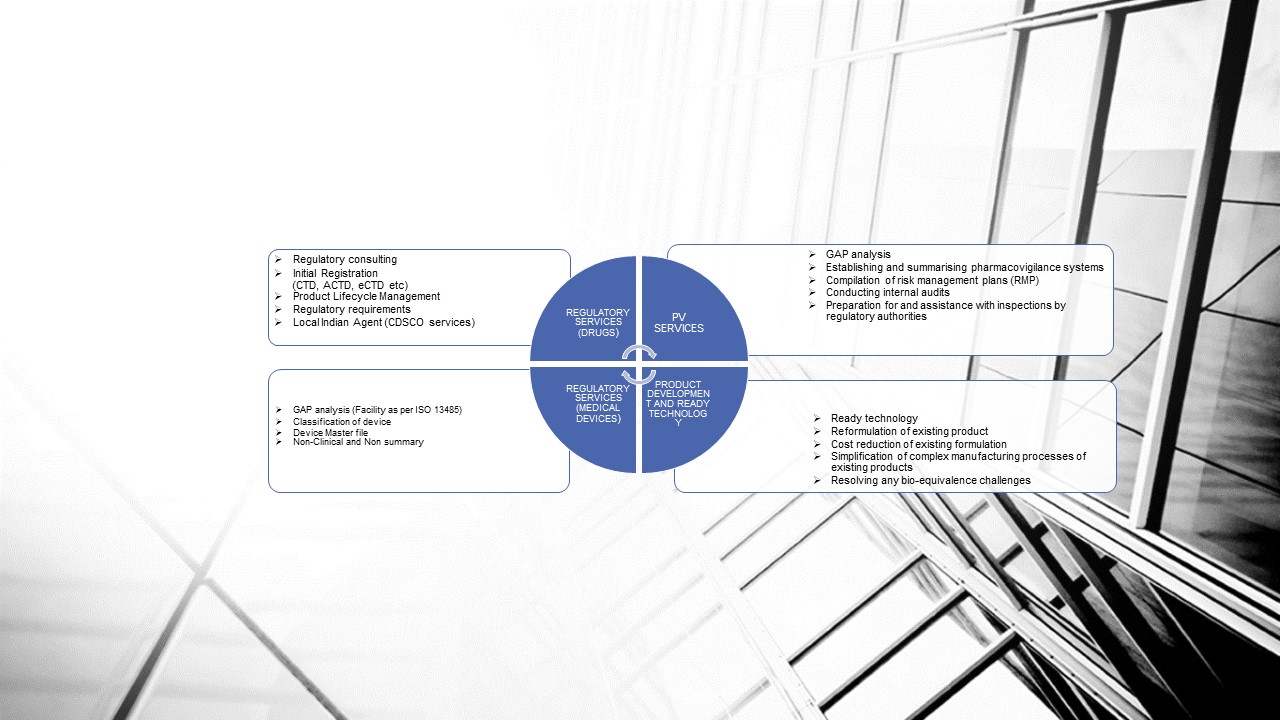
REGULATORY SERVICES (Medical Devices)
‘Medical device’ means any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination, for human beings, for one or more of the specific medical purpose(s) of:
- diagnosis, prevention, monitoring, treatment or alleviation of disease,
- diagnosis, monitoring, treatment, alleviation of or compensation for an injury,
- investigation, replacement, modification, or support of the anatomy or of a physiological process,
- supporting or sustaining life,
- control of conception,
- disinfection of medical devices
- providing information by means of in vitro examination of specimens derived from the human body;
and does not achieve its primary intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its intended function by such means.
Note: Products which may be considered to be medical devices in some jurisdictions but not in others include:
- disinfection substances,
- aids for persons with disabilities,
- devices incorporating animal and/or human tissues,
- devices for.in-vitro fertilization or assisted reproduction technologies
Medwisdom Provide the following services:
- Classification of medical device
- Preparation of technical master file
- Clinical and non-clinical expert report of medical device and in vitro diagnostic.
Expert advice covering a wide range of products, from simple bandages to the most sophisticated life-supporting products, the medical devices sector plays a crucial role in the diagnosis, prevention, monitoring, and treatment of diseases and the improvement of the quality of life of people suffering from disabilities.